 Salamanders are interesting creatures. When attacked by a predator, they will shed their tail and flee. The tail will wriggle around on its own for long enough to hopefully distract the predator and allow the salamander to escape. Fortunately, losing a tail is not a huge bother for the little guy; he’ll just grow back a new one.
Salamanders are interesting creatures. When attacked by a predator, they will shed their tail and flee. The tail will wriggle around on its own for long enough to hopefully distract the predator and allow the salamander to escape. Fortunately, losing a tail is not a huge bother for the little guy; he’ll just grow back a new one.
Outside a select group of amphibians, the ability of vertebrate species to regenerate complex organs and body parts is extremely limited. In humans, it is almost non-existent (with the notable exception of the liver). But what cannot be done by nature, we can achieve though science! Or at the least, it may be possible in the future…
Regenerative medicine is a relatively new field that is creating new organs and new possibilities. Last month, it was reported in The Lancet that a 10 year old Swedish girl received a successful transplant of a vein grown in a lab from her own stem cells1. The girl was suffering from a liver portal vein obstruction, a potentially fatal disease. If she had been an adult, a vein would have been removed from her neck or leg and used as a replacement. However in the case of a still growing child, this procedure carries unacceptable difficulties. Instead, they grew one.
A vein from a deceased donor was stripped of all its cells and the remaining scaffold “seeded” with the girl’s own stem cells. This scaffold was then placed in a “bioreactor” which bombarded the structure with chemicals necessary for programming the stem cells to develop in a specific way. This allowed the new vein to mature before transplantation. Following a second similar procedure the girl returned to full health.
When it comes to organ regeneration experts have identified 4 levels of complexity:
- Simple flat structures such as skin and endothelium.
- Tubular structures such as blood vessels and tracheae.
- “Hollow” organs such as bladders.
- Organs with complex substructures such as kidneys or lungs.
The Lancet report is just the latest example of engineered organs being transplanted into humans. Indeed, similar results have previously been achieved with bladders, urethras and tracheae2-4. These manufactured organs have the advantage over normal transplants of being wholly compatible with the patient’s immune system, rendering the need to take dangerous immunosuppressants moot. However, whilst the scaffold technique is useful as a proof-of-concept, in practice problems can arrise. The scaffold may not be a suitable size, causing it to mismatch with surrounding tissue. The growth of cells on the scaffold may be uneven or insufficient. Furthermore, no group have yet created a level D organ using this method.
These challenges have led scientists to look for other options. 3D printing is an industrial manufacturing technique that has recently adapted and expanded into biomedical applications. A 3D printer creates objects by the process of “additive manufacturing”. The printer deposits layers of material (usually small, discrete pieces of metal or plastic) on top of one another onto a flat surface according to a programmed input. These layers are then melted together to form a complete object. This process is used to form many complex objects including machine components, jewellery and toys.
[youtube http://www.youtube.com/watch?v=hk8u5CefYsc]
Very recently, 3D printers have become capable of laying down material at the micrometre resolution (one-thousandth of a millimetre), a scale which allows accuracy at the level of a single cell. Biologists have since been experimenting with using 3D printers to print human organs. Small clumps of cells are bound together with a rapidly-degrading film that allows these clumps to be printed three-dimensionally in a fairly structured manner. The cells are then deposited in a shape and structure similar to a specific organ. Whilst the arrangement of the printed cells is not quite the same as a naturally occurring organ, the cells are able to detect their environment and re-organise themselves into a more conventional structure. One example is shown below.
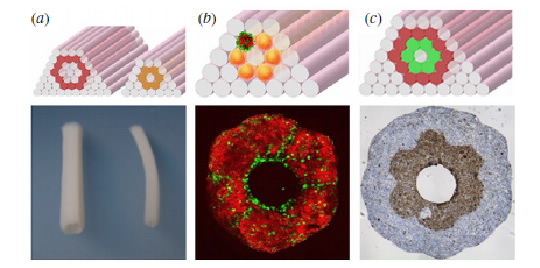
The printed cells used for this technique can either be specific cell types as shown above or more generic stem cells. Once 3D constructs have been formed, the tissue is maturated in a bioreactor to form a stable organ, similar to previous techniques. The now consolidated organ is essentially functional and in theory could be used for transplantation. However, such is the novelty of this technique that it hasn’t yet been put into practice.
Bioprinting has huge strengths over similar techniques including customisation, flexibility and reproducibility whilst not relying on donor material. In theory any cell type, body structure or tissue may be used in this technique providing the correct design and the correct type of “ink” is used. Even bone may be printable using a combination of osteogenic cells and temporary connective tissue replacements.
The potential for this technology is vast. Imagine if we were to bear the maximum fruits of regenerative research. Organ donor waiting lists would become a thing of the past. No more children on dialysis. No more diabetics on insulin. No more hormone disorders (we could replace faulty hormone-releasing organs). Men and women injured in wars or in car crashes would have limbs replaced or healed.
And what about non-therapeutic uses? Want a breast implant? No need to implant a silicon or saline bag – just add more breast. The number of animals killed for research purposes would greatly decrease. When you can test drugs and conduct experiments on organs grown in a lab, the need to extract these organs from animals would no longer exist.
Whatever the possibilities may be, research is still at an early stage. Printers of adequate sophistication cost in the range of tens of thousands of pounds, choking research in this area. We can only hope that as prices collapse, so will the barriers to progress.
Post by Chris Logie
References
1. Olausson M. et al. (2012). “Transplantation of an allogeneic vein bioengineered with autologous stem cells: a proof of concept study.” Lancet 380 9838:230-7.
2. Atala, A. et al (2006). “Tissue-engineered autologous bladders for patients needing cystoplasty”. Lancet 367 9518:1241-6.
3. Raya-Rivera, A. et al. (2011). “Tissue-engineered autologous urethras for patients who need reconstruction: an observational study.” Lancet 377 9772:1175-82.
4. Macchiarini, P. (2009). “Clinical transportation of a tissue-engineered airway.” Lancet 372 9655:2023-30.
5. Jakab, K. (2010). “Tissue engineering by self-assembly and bio-printing of living cells.” Biofabrication 2 2:022001
6. Fedorovich, N. E. (2009). “Organ printing: the future of bone regeneration.” Trends Biotechnol 29 12:601-6


 This rift is exemplified by the shocking fact that there is only one British Member of Parliament out of 650 that has a scientific, research-based PhD (Lib Dem MP for Cambridge Julian Huppert, Biological Chemistry, in case you’re wondering). Similarly in the United States, 3 in 435 people in the House of Representatives have a non-medical, scientific background. And considering Prof. Nutt’s dismissal, it seems that scientists are seen by politicians as commodity experts whose advice can be cherry-picked for a bit of ‘policy-based evidence-making’. Winston Churchill once said that science should be ‘on tap but not on top’. But what’s stopping us scientists from getting properly involved in politics?
This rift is exemplified by the shocking fact that there is only one British Member of Parliament out of 650 that has a scientific, research-based PhD (Lib Dem MP for Cambridge Julian Huppert, Biological Chemistry, in case you’re wondering). Similarly in the United States, 3 in 435 people in the House of Representatives have a non-medical, scientific background. And considering Prof. Nutt’s dismissal, it seems that scientists are seen by politicians as commodity experts whose advice can be cherry-picked for a bit of ‘policy-based evidence-making’. Winston Churchill once said that science should be ‘on tap but not on top’. But what’s stopping us scientists from getting properly involved in politics? The main thing scientists have got going for them if they fancy residing in Downing Street is the doctrine of science itself. As Carl Sagan (the American Brian Cox of his time) said, “science is a way of thinking much more than it is a body of knowledge”. The knowledge bit isn’t bad either and it lasts far beyond the sell-by date of parties’ policies. The logic that underlies the analysis of data gathered from random, blinded, controlled trials is the perfect way of objectively testing different policies. And we’ve got buckets of that logic to share with our MPs.
The main thing scientists have got going for them if they fancy residing in Downing Street is the doctrine of science itself. As Carl Sagan (the American Brian Cox of his time) said, “science is a way of thinking much more than it is a body of knowledge”. The knowledge bit isn’t bad either and it lasts far beyond the sell-by date of parties’ policies. The logic that underlies the analysis of data gathered from random, blinded, controlled trials is the perfect way of objectively testing different policies. And we’ve got buckets of that logic to share with our MPs. Turns out the obstacle in this film would be the fact I like crisps, drink too much wine and avoid exercise. So what’s a girl (or boy) to do? There must be a quick fix answer, surely. Well, my fascination with everything that allows you to procrastinate on the internet has led me to some rather shady websites that peddle weight loss pills that promise you can lose a stone in two weeks. With this in mind I decided to look into the ‘science’ of how diet pills work – after all if they look like a real medicine they must work like a real medicine. Maybe I’m just thinking of the placebo effect there. Anyway these are some types of pills I found that you can pop à la the internet:
Turns out the obstacle in this film would be the fact I like crisps, drink too much wine and avoid exercise. So what’s a girl (or boy) to do? There must be a quick fix answer, surely. Well, my fascination with everything that allows you to procrastinate on the internet has led me to some rather shady websites that peddle weight loss pills that promise you can lose a stone in two weeks. With this in mind I decided to look into the ‘science’ of how diet pills work – after all if they look like a real medicine they must work like a real medicine. Maybe I’m just thinking of the placebo effect there. Anyway these are some types of pills I found that you can pop à la the internet: Incidentally, açaí berries are the fruit world’s answer to
Incidentally, açaí berries are the fruit world’s answer to  A popular pill ingredient is p57 derived from a plant called Hoodia. Although there is evidence to suggest it can affect signalling in the brain and suppress the appetite of rats, the rats in question were injected with the molecule directly into their brain. So unless you’re going to inject your brain with p57, (to be clear no one should EVER do that), I’d take that with a pinch of salt.
A popular pill ingredient is p57 derived from a plant called Hoodia. Although there is evidence to suggest it can affect signalling in the brain and suppress the appetite of rats, the rats in question were injected with the molecule directly into their brain. So unless you’re going to inject your brain with p57, (to be clear no one should EVER do that), I’d take that with a pinch of salt.


