Using fat to treat obesity and obesity-related conditions, such as type II diabetes, may sound like a strange idea. Nevertheless, this is exactly what scientists are working to achieve. So the question is, why?
There are in fact two types of fat in the body. The more familiar of the two – white adipose tissue (WAT) – is the tissue we refer to colloquially as “fat” and associate with gaining weight. The second, and lesser known variety of fat, is brown adipose tissue (BAT). This unique tissue is densely innervated by the body’s sympathetic nervous system (SNS – initiator of the “fight or flight” response) and is well supplied by blood vessels. Originally believed only to exist in small mammals and human infants. BAT is used to produce heat without the need for shivering. This is particularly important for these organisms as a way of maintaining their core temperature in mild cold conditions.
The key to BAT’s ability to produce heat is a special protein called uncoupling protein 1 (UCP1), which is housed within the large number of mitochondria found in this tissue. In general, mitochondria act as the “powerhouses” of all cells, producing energy in the form of ATP which allows the cell to perform all its required functions.
However, in cold conditions, sensors on the skin send a signal to the part of the brain responsible for regulating body temperature. The brain, in turn, sends a message to BAT via the SNS, releasing noradrenaline and stimulating UCP1. Upon activation, UCP1 is able to “override” the usual ATP-synthesising function of BAT mitochondria, instead releasing energy as heat.
Importantly, this process of heat production requires a significant amount of energy to achieve. As the main fuel used by BAT are fat molecules, such as lipids and fatty acids, the idea that BAT could potentially be used to help reduce body weight in obesity is not as ludicrous as it may first appear. This is supported by the finding that both UCP1- and BAT-deficient mice models display increased weight gain.
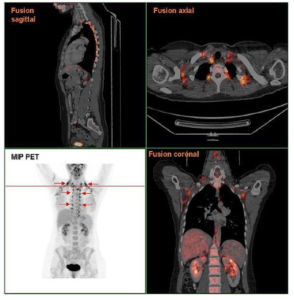 Despite evidence of the role BAT plays in controlling body weight in experimental rodent models, BAT was originally believed to be absent in adult humans. More recently, however, the use of radioactive tracer PET and CT scans have demonstrated the presence of functional BAT in adults in mild cold conditions using large cohorts of patients. Further analysis of PET and CT data has also uncovered an inverse association between BAT activity and BMI, with lower levels of activity in patients with severe obesity. Though the average human adult is estimated to possess just 50–80g of BAT, this mass is believed to use up to 20% of our daily energy intake, making BAT a desirable candidate to aid weight loss in obesity.
Despite evidence of the role BAT plays in controlling body weight in experimental rodent models, BAT was originally believed to be absent in adult humans. More recently, however, the use of radioactive tracer PET and CT scans have demonstrated the presence of functional BAT in adults in mild cold conditions using large cohorts of patients. Further analysis of PET and CT data has also uncovered an inverse association between BAT activity and BMI, with lower levels of activity in patients with severe obesity. Though the average human adult is estimated to possess just 50–80g of BAT, this mass is believed to use up to 20% of our daily energy intake, making BAT a desirable candidate to aid weight loss in obesity.
BAT has also been shown to express high levels of the glucose-transporter protein and displays comparable glucose uptake to muscle in response to insulin. Given that a key risk factor for type II diabetes is being overweight, and that this disorder is characterised by high blood glucose and insulin resistance, targeting BAT as a method of treatment is also under investigation. Notably, insulin-activated glucose uptake into BAT is significantly lower in those with obesity compared to subjects of a normal weight.
Given the mechanism of BAT activation discussed above, as well as the observation during PET and CT scans that BAT activity is inhibited when human subjects are warmed, the simplest way to stimulate this tissue for therapeutic reasons may be with the use of cold temperatures. In support of this theory, a small study involving healthy Japanese volunteers, who were repeatedly exposure to mild cold stimuli over 6 weeks, reported increased BAT and resulted in a loss of body fat in subjects. In another study, exposing healthy volunteers with active BAT to short-term mild cold led to increased insulin sensitivity.
 A second potential method of stimulating BAT with the aim to treat obesity or type II diabetes is via the SNS, specifically through beta-adrenergic receptors (βARs). It should be noted that anti-obesity medications which target these receptors have been tested in the past. However, these attempts failed due to unspecific activation of βARs throughout the body – particularly the heart – resulting in serious side effects, including increased blood pressure and heart rate. Nevertheless, the use of more specific βAR agonists have been used successfully to increase BAT activity and lessen obesity and insulin resistance in rodent models, without such side effects.
A second potential method of stimulating BAT with the aim to treat obesity or type II diabetes is via the SNS, specifically through beta-adrenergic receptors (βARs). It should be noted that anti-obesity medications which target these receptors have been tested in the past. However, these attempts failed due to unspecific activation of βARs throughout the body – particularly the heart – resulting in serious side effects, including increased blood pressure and heart rate. Nevertheless, the use of more specific βAR agonists have been used successfully to increase BAT activity and lessen obesity and insulin resistance in rodent models, without such side effects.
Alternatively, it may be possible to target the thermoreceptors on the skin responsible for sensing cold, known as TRPs. As cold exposure cannot be controlled easily, activating these receptors artificially may provide a more efficient way of using BAT to treat obesity or type II diabetes. Interestingly, there are already a number of foods which activate TRPs, including menthol in mint and capsaicin in chili peppers. Animal and human studies of capsinoids – non-pungent analogues of capsaicin – have demonstrated that these compounds increase active BAT and reduce body fat.
Despite sounding rather contradictory, adipose tissue may prove useful in the treatment of obesity and the obesity-related disorder, type II diabetes. Unlike its white counterpart, BAT actually uses up lipids and glucose from the body as fuel in order to produce heat. However, in doing so, evidence suggests this can also result in weight loss, reduced insulin resistance and lowered blood glucose, making it a potential treatment for obesity and type II diabetes. Possible methods of activating BAT for this purpose may include cold stimuli or agonists which target βAR or TRP receptors. It is still early days for this treatment possibility but the idea certainly isn’t as strange as it may first appear.
Post by: Megan Barrett
Save
Save